Contact
PD Dr. med. Samir Sarikouch
Clinical Trial Director
e-mail: sarikouch.samir(at)mh-hannover.de
For Physicians
As the number of humans with congenital heart defects is growing and the population in the EU is ageing in general, thereby leading to higher health costs, reduction of costs for heart valve replacement could compensate for this to some extent. A reduction of one percent of total costs for cardiovascular diseases, which seems realistic given the high numbers of heart valve operations (60.000 per yr., EACTS-database), would represent savings of about more than 1 billion Euros.
Biological valve replacement is the common treatment in advanced pulmonary valve (PV) and aortic valve (AV) disease. Accelerated degeneration of biological allo- and xenovalves is partially attributed to remaining cells within the valve tissue. Preserved antigenicity induces a chronic inflammatory response with subsequent valve failure. In addition, all described grafts have a limited acceptance in patients that are still growing. Immunological responses are avoided by the use of the patient’s own pulmonary valve in replacement of the diseased aortic valve, as in the Ross operation. The pulmonary autograft is also advantageous, as it has been shown to grow along with a child, resulting in fewer re-operations. Factors contributing to a limited acceptance of this procedure include operation complexity and the replacement requirement of both aortic and pulmonary valves. Moreover, cryopreserved allografts undergo degenerative processes which are the leading cause for re-operation after 10 years.
As a result, implantation of acellular or reseeded heart valves with patients’ own cells may solve the immune response problems and facilitate in-vivo graft remodeling.
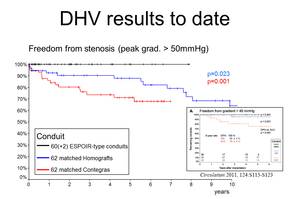
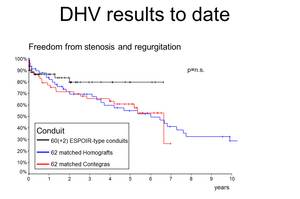
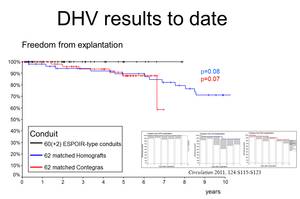
Over the last decade, tissue engineering (TE) has become a promising strategy to obtain such valves. Early clinical experience with decellularized homografts, proceeded by methods of TE prior to implantation in pulmonary position, showed unlike conventional homografts and xenografts, improved freedom from explantation, provided low gradients in follow-up and exhibited adaptive growth.
Figures 1-3 delineate the results of DHV implanted in pulmonary position that have been obtained so far. The black curves represent the ESPOIR-type decellularized heart valve, blue represents cryopreserved conventional homografts and the red bovine jugular vein conduits, Contegra«.
In sheep, a model considered to be the standard in predicting calcification of biological heart valves, decellularized AV demonstrated superior durability as compared to unprocessed conventional allografts in long-term experiments.
As a consequence, AV replacements with TE grafts now have reached pre-clinical level and at the MHH, several decellularized homografts have been implanted in selected complicated cases.
References
Cebotari S, Tudorache I, Ciubotaru A, Boethig D, Sarikouch S, Goerler A, Lichtenberg A, Cheptanaru E, Barnaciuc S, Cazacu A, Maliga O, Repin O, Maniuc L, Breymann T, Haverich A. Use of fresh decellularized allografts for pulmonary valve replacement may reduce the reoperation rate in children and young adults: early report. Circulation. 2011 Sep 13;124(11 Suppl):S115-23.
Baraki H, Tudorache I, Braun M, H÷ffler K, G÷rler A, Lichtenberg A, Bara C, Calistru A, Brandes G, Hewicker-Trautwein M, Hilfiker A, Haverich A, Cebotari S. Orthotopic replacement of the aortic valve with decellularized allograft in a sheep model. Biomaterials 2009; 30:6240–6246.